Palm Biology Research at Fairchild
Fairchild Tropical Botanic Garden's Center for Tropical Plant Conservation has an active research program dealing with different aspects of palms: their microscopic structure, DNA, taxonomy, developmental anatomy, etc.
Researchers
- Dr. Jack B. Fisher – Senior Research Scientist
- Dr. P. Barry Tomlinson – Research Associate
- Dr. Scott Zona – Palm Biologist
- Dr. Carl Lewis
Research Projects
- Work towards a second edition of Anatomy of Palms, including Plates A-C
- The Taxonomy of Pseudophoenix
- Palm root biology
- Structure and function of climbing palms
- The Palms of New Guinea
- Developmental anatomy of ruminate endosperm in palm seeds
- Significance of starch in pollen of palms
- Significance of raphid crystals in embryos of palms
- Palm DNA Bank
- Long-term Study of Coccothrinax argentata from Florida
- Palm Family Phylogeny
- Recent publications in palm anatomy from FTBG
- Additions to "A review of animal-mediated seed dispersal of palms" by Scott Zona
- Survey of Cyanogenesis in Palms (Arecaceae) by Scott Zona and Carl Lewis (PDF document)
- Heart of Palm Usage
Second Edition of Anatomy of Palms
Work on a new (second) edition of Vol 2. Palmae in the series “The Anatomy of the Monocotyledons” Oxford University Press is now in progress. A grant proposal is under review by the National Science Foundation. Three Plates of figures are presented below to show how the project will document genera and species of palms.
NSF Proposal Title and Summary (doc file)
NSF Proposal References (pdf file)
Characters for lamina anatomy (pdf file)
Plate A.
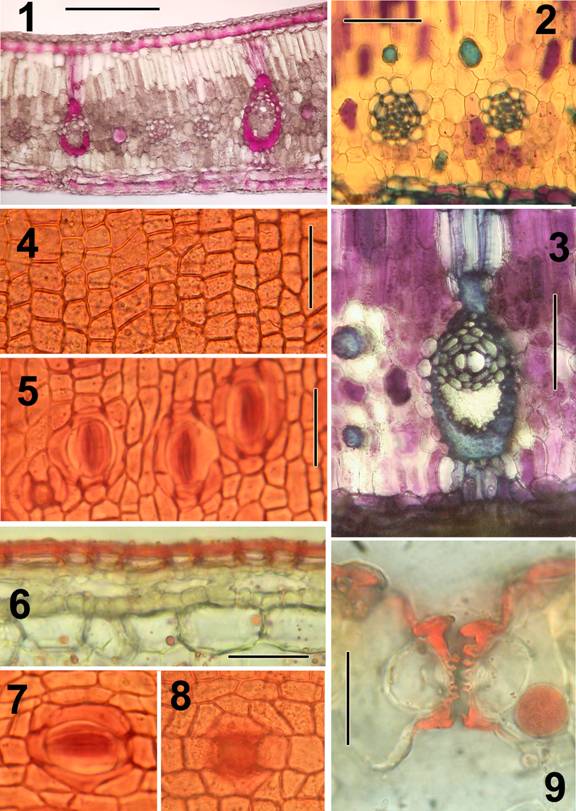 Plate A: Nypa fruticans (the mangrove palm).
Plate A: Nypa fruticans (the mangrove palm).
Nypa fruticans is the sole representative of the subfamily Nypoideae and appears to have a basal position in palm phylogeny (Asmussen et al. 2000).
However, it is also unique among palms in its mangrove habitat.
Deciphering possible ancestral features from those that are ecological adaptations (e.g., stomatal structure) requires extensive comparative study.
Representative illustrations indicating type of preparations produced by the rapid methods described.
All preparations were made without tissue imbedding.
Lamina not desilicified.
Sections mounted in glycerine:water (1:1) after treatments indicated.
Sections were cut at 20-40 µm thickness; thinner sections can be cut after desilicification in 50% HF.
Figures:
1.) TS Lamina; lignified tissues stained red (stained in phloroglucinol:HCl.
2.) TS Lamina; small vascular bundles and fiber bundles (0.5% aqueous toluidine blue after bleaching in 5% Na hypochlorite).
3.) TS major vein of lamina (same treatment as in Fig. 2).
4.) Adaxial epidermis in surface view from macerated material (stained in 1% safranin in 70% ethanol).
5.) Abaxial epidermis in surface view with stomates (same treatment as in Fig. 4).
6.) TS adaxial surface layers; epidermis wholly cutinized (bleached in 5% Na hypochlorite; stained in 1% Sudan IV in 95% ethanol).
7.) Stomate in surface view (same treatment as in Fig. 4).
8.) Pore or aborted stomata from adaxial surface in surface view (same treatment as in Fig. 4).
9.) TS stomatal apparatus with diagnostic ribbed guard cells, appearing notched
(lamina bleached in 5% Na hypochlorite; stained in 1% Sudan IV in 95% ethanol).
Bar = 200µm in Fig. 1; = 100 µm in Fig. 2,3; = 50 µm in Fig. 4; = 20µm in Fig. 5-8; = 25 µm in Fig. 9.
Plate B.
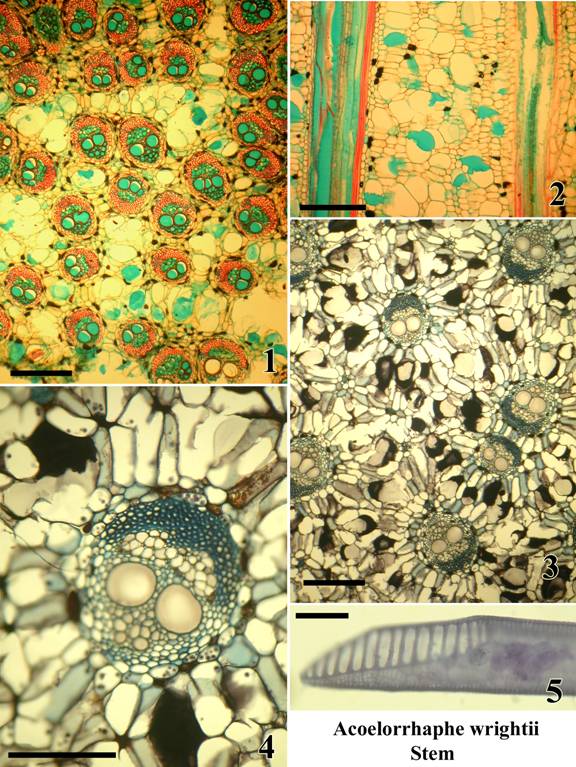 Plate B: Acoelorrhaphe wrightii. Stem.
Plate B: Acoelorrhaphe wrightii. Stem.
A monotypic genus of clustering palms in subfamily Coryphoideae – tribe Corypheae;
native to South Florida, Mexico and the West Indies.
Microtome sections are mounted permanently.
Figures:
1.) TS upper stem sub-periphery (safranin and alcian green).
2.) LS upper stem sub-periphery (safranin and alcian green).
3.) TS lower stem center (tannic acid and resorcin blue)
4.) TS single vascular bundle in lower stem center (tannic acid and resorcin blue), two wide metaxylem vessels.
5.) Metaxylem vessel isolated by maceration with scalariform perforation plate (Delafield’s haematoxylin).
Bar = 400µm in Fig. 1,3; = 200 µm in Fig. 2,4; = 50 µm in Fig. 5
Plate C.
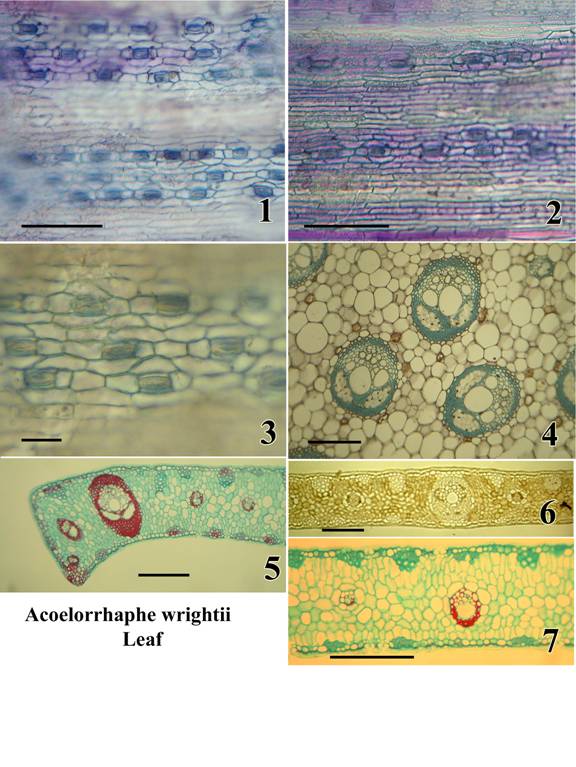 Plate C: Acoelorrhaphe wrightii. Leaf.
Plate C: Acoelorrhaphe wrightii. Leaf.
A monotypic genus of clustering palms in subfamily Coryphoideae – tribe Corypheae;
native to South Florida, Mexico and the West Indies.
Figures:
1.) Abaxial epidermis in surface view; sunken stomatal level in focus; non-stomatal level out of focus (wet preparation stained with toluidine blue).
2.) Adaxial epidermis in surface view (same stain).
3.) Stomata in surface view; wax layer out of focus (same stain).
4.) TS, petiole; note two phloem strands per bundle (permanent preparation, tannic acid and resorcin blue).
5.) TS margin of lamina segment (permanent preparation, safranin and alcian green).
6.) TS lamina segment (unstained, mounted in Karo syrup).
7.) TS lamina segment (permanent preparation, safranin and alcian green).
Bar = 100µm in Fig. 1,2; = 50 µm in Fig. 3; = 500µm in Fig. 4; = 500 µm in Fig. 5,6,7.
The Taxonomy of Pseudophoenix

|
| Pseudophoenix vinifera
in the Dominican Republic (photo S. Zona) |
Quintessentially Caribbean, Pseudophoenix is a small genus of attractive, medium-sized palms. Four species occur in the Caribbean Basin; they are P. sargentii, P. vinifera, P. ekmanii, and P. ledeniana. Florida's species, Pseudophoenix sargentii, occurs only in the Florida Keys, where it is severely endangered, and is the subject of an intensive conservation and reintroduction program. While conservation activities are vitally important, the foundation for any conservation activities must be solid taxonomy. The identification, natural distribution, classification, and inter-relationships of Pseudophoenix were studied by Palm Biologist Dr. Scott Zona, who found that Pseudophoenix sargentii comprises just one, highly variable species (it was previously believed to have several named varieties and subspecies).
Zona, S. 2002.
A revision of Pseudophoenix.
PALMS 46: 19-38.
Palm Root Biology
 |
| Fungal symbiont with cells of root of Chamaedorea (coils). (photo J.B. Fisher) |
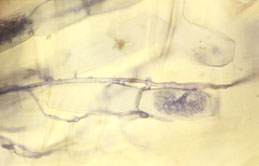 |
| Fungal symbiont with cells of root of Neonicholsonia (arbuscule). (photo J.B. Fisher) |
One-half of every palm is underground. The private lives of palm roots are largely unknown to botanists, many of whom study crop plants or temperate zone plants. In an innovative program initiated at Fairchild Tropical Botanic Garden, Dr. Jack Fisher is examining the little-known world of palm root biology.
Dr. Fisher is studying how palm roots grow and function. Palms, like many plants, form symbiotic partnerships with soil fungi (mycorrhizae), but how do these partnerships form in new seedlings?
How do roots and fungi find each other?
How long does the partnership last, and how do both parties benefit?
These are some of the questions currently under study by Dr. Fisher, in collaboration with Dr. K. Jayachandran of Florida International University.
Fisher, J. B. & K. Jayachandran. 1999.
Root structure and arbuscular mycorrhizal colonization of the palm Serenoa repens under field conditions.
Plant and Soil 217: 229-241.
Structure and Function of Climbing Palms
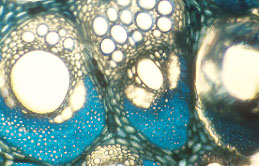
|
| Magnified view of two vascular bundles in cross-section with a wide vessel in each bundle of Calamus.
(photo J.B. Fisher) |
Some palms have flexible stems and climb as vines on other trees in the rainforest. These palms are the rattans of commerce and have stems longer (taller) than California redwoods, the tallest known trees.
How do these palms bring water from their roots to their leaves?
To answer this question, Fairchild Tropical Botanic Garden Senior Research Scientist Dr. Jack Fisher is examining the three-dimensional arrangement of water-conducting tissues in the stems of climbing palms.
This project is being undertaken in collaboration with Harvard University botanist Dr. P. B. Tomlinson and other colleagues.
Fisher, J.B., H.T.W. Tan, and L.P.L Toh. 2002.
Xylem of rattans: vessel dimensions in climbing palms.
Amer. J. Bot. 89: 196-202.
Tomlinson, P.B., J.B. Fisher, R.E. Spangler, and R.A. Richer. 2001.
Stem vascular architecture in the rattan palm Calamus (Arecaceae – Calamoideae – Calaminae).
Amer. J. Bot. 88: 797-709.
Tomlinson, P. B., J. B. Fisher. 2000.
Stem vasculature in climbing Monocotyledons: A comparative approach, pp. 89-97.
in K. L. Wilson, D. A. Morrison (eds).
Monocotyledons: Systematics and Evolution.
Melbourne, Australia.
The Palms of New Guinea
 |
| The colorful fruits of Drymophloeus oliviformis in Irian Jaya, New Guinea. (photo S. Zona) |
The island of New Guinea is home to an untold diversity of palms, including some of our best-loved ornamental palms, such as Ptychosperma macarthurii and Drymophloeus oliviformis. Palm Biologist Dr. Scott Zona was invited to join a team of researchers headed by Drs. Bill Baker and John Dransfield of the Royal Botanic Gardens, Kew, England, to identify and document all the palms of New Guinea. Dr. Zona will contribute taxonomic accounts of the genera of the Ptychospermatinae, which include Brassiophoenix, Drymophloeus, Ptychosperma, and Ptychococcus.
Zona, S. & F. B. Essig. 1999.
How many species of Brassiophoenix?
Palms (formerly Principes) 43: 45-48.
Zona, S. 1999.
A revision of Drymophloeus (Arecaceae: Arecoideae).
Blumea 44: 1-24.
Zona, S. 2000.
A personal history of Drymophloeus in New Guinea.
PALMS 44: 184-186.
Developmental Anatomy of Ruminate Endosperm in Palm Seeds
 |
| Longisection through seed of Adonidia merrillii showing ruminate endosperm intruded by the light brown testa. |
Many palm seeds have ruminate endosperms. The condition is characterized by in-growths of the seed coat (ruminations) that penetrate the endosperm (the food storage tissue in a seed). It is an important taxonomic character used by botanists to identify different species of palms.
Preliminary studies show that there is more than one way in which this condition develops, which suggests that not all ruminations are homologous. Careful study of the development by Dr. Scott Zona may reveal previously unsuspected differences among different palm lineages.
Zona, S. 2003.
Endosperm condition and the paradox of Ptychococcus paradoxus.
Telopea 10: 179-185.
Significance of starch in pollen of palms
Starchy pollen appears to be a characteristic
of the Commelinoid (Commelinid) monocots
(orders Arecales, Poales, Commelinales, and Zingiberales).
A survey of 31 palms found starchy pollen
(as indicated by IKI staining) in all but four species.
The gene that controls starch synthesis in pollen and endosperm is the same,
the granule-bound starch synthase, but its expression is tissue specific.
Although the gene is expressed in the pollen of palms,
it is not expressed in palm endosperms,
which store lipids or carbohydrates other than starch.
Zona, S. 2001.
Starchy pollen in Commelinoid monocots.
Annals of Botany 87: 109–116.
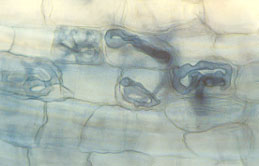
Significance of raphid crystals in embryos of palms
 |
| Raphides from the embryo of Pinanga negrosensis, viewed through crossed polarized light. Scale bar equals 0.1 mm. |
Raphides, bundles of needle-shaped crystals of calcium oxalate,
have been known from palm embryos since 1891,
but there has never been a systematic survey of their occurrence in palm embryos.
This survey of 148 taxa found that raphides are neither randomly
nor evenly distributed across the palm family.
Raphides are present in the subfamilies Coryphoideae and Ceroxyloideae,
but they are not common.
In contrast, raphides are a common feature of embryos of taxa of the Arecoideae.
They are absent from the embryos of the Calamoideae and Phytelephantoideae.
Zona, S. 2004.
Raphides in palm embryos and their systematic distribution.
Annals of Botany 93: 415–421
Palm DNA Bank
The recent advances in gene sequencing are allowing molecular biologists to study palm evolution with a degree of precision never before possible. The Garden's living palm collection is a invaluable resource for researchers looking at palm DNA, but living collections are not permanent. Losses due to disease, lightning, accident, and theft are a reality we face, but by extracting a sample of DNA and preserving it in an ultra low freezer, our palm collection has a scientific life span longer than the life of the individual palm. With assistance from a a grant from the Stanley Smith Horticultural Trust, Dr. Carl E. Lewis is creating a DNA bank from the palms at FTG. He is using the samples in his own work on palm molecular evolution, and he shares the samples with researchers all over the world.
Long-Term Study of Coccothrinax argentata from Florida

|
| Dr. Scott Zona examining six-year old Coccothrinax argentata plants.
(photo J. B. Fisher) |
Did you every wonder why our native Silver Thatch Palm (Coccothrinax argentata) is short in stature in Broward and Miami-Dade Counties and tall in Monroe County? Are the palms genetically distinct, or is the difference in stature simply the result of differences in soil types? Palm Biologist Dr. Scott Zona is conducting a long-term common garden experiment to examine this question. In 1993, he collected seeds from populations of Coccothrinax argentata from throughout its Florida range. The plants that grew from these seeds have been planted out in a uniform environment and will be monitored over the next two decades for differences in growth and stature. For this project, Dr. Zona is collaborating with the staff of the Montgomery Botanical Center.
Palm Family Phylogeny
Dr. Carl E. Lewis joins a team of researchers from Cornell University, the Royal Botanic Gardens, Kew (UK), and the Royal Veterinary and Agricultural University (Denmark) to study and uncover the palm family tree, or phylogeny. This unprecedented effort to discern the relationships among all the genera of palms using molecular, anatomical, cytological, palynological, and morphological data will be a major step forward in understanding the evolutionary history of this important group of plants.
Recent Publications in Palm Anatomy (pdf files)
Fisher, J. B., G. Angeles, F. W. Ewers, and J.
López-Portillo. 1997.
Root pressures in tropical lianas and other woody plants.
Int. J. Plant Sci. 158: 44-50.
Fisher, J. B., J. N. Burch, and L.R. Noblick. 1996.
Stem structure of the Cuban belly palm (Gastrococos crispa).
Principes 40: 125-128.
Fisher, J. B., K. Jayachandran. 1999.
Root structure and arbuscular mycorrhizal colonization of the palm Serenoa repens under field conditions.
Plant and Soil 217: 229-241.
Fisher, J. B. & K. J. Maidman. 1999.
Branching and architecture in palms: Value for systematics.
Mem. New York Bot. Gard. 83: 35-46.
Fisher, J.B., H.T.W. Tan, and L.P.L Toh. 2002.
Xylem of rattans: vessel dimensions in climbing palms.
American Journal of Botany 89: 196-202.
Lewis, C. E. and Jeff J. Doyle. 2002.
A phylogenetic analysis of palm tribe Areceae using two low-copy nuclear genes.
Plant Systematics and Evolution 236:1-17.
Lewis, C. E. 2002.
A phylogenetic study of palm subtribe Oncospermatinae based on morphological characters.
Brittonia 54:78-91.
Lewis, C. E. and Jeff J. Doyle. 2001.
Phylogenetic utility of the nuclear gene malate synthase in the palm family (Arecaceae).
Molecular Phylogenetics and Evolution 19:409-420.
Lewis, C. E., C. B. Asmussen, and W. J. Baker.
2000.
DNA and Palm Evolution.
Palms 44: 19-24.
Lewis, C. E. and N. Martinez. 2000.
Identity of the Hyophorbe palms at the Botanical Garden of Cienfuegos, Cuba.
Palms 44: 93-97.
Lewis, C. E. & S. Zona. 2000.
A survey of cyanogenesis in palms (Arecaceae).
Biochem. Syst. Ecol. 28: 219-228.
Tomlinson, P. B. & J. B. Fisher. 2000.
Stem vasculature in climbing Monocotyledons: A comparative approach, pp. 89-97.
in K. L. Wilson & D. A. Morrison (eds). Monocotyledons: Systematics
and Evolution. CSIRO, Melbourne, Australia.
Tomlinson, P.B., J.B. Fisher, R.E. Spangler, and R.A. Richer. 2001.
Stem vascular architecture in the rattan palm Calamus (Arecaceae – Calamoideae – Calaminae).
American Journal of Botany 88: 797-709.
Zona, S. 1997.
The genera of Palmae (Arecaceae) in the southeastern United States.
Harvard Papers in Botany 11: 71-107.
Zona, S. 1998.
Chuniophoenix in cultivation.
Principes. 42: 198-200.
Zona, S. 1999.
New perspectives on generic limits and relationships in the Ptychospermatinae (Palmae: Arecoideae).
Mem. New York Bot. Gard. 83: 255-263.
Zona, S. 1999.
A revision of Drymophloeus (Arecaceae: Arecoideae).
Blumea 44: 1-24.
Zona, S. 2000a.
Arecaceae, p. 95–123,
in Flora of North America Editorial Committee (eds.), Flora of North America, vol. 22. Magnoliophyta: Alismatidae, Arecidae, Commelinidae (in part), and Zingiberidae.
Oxford Univ. Press, New York.
Zona, S. 2000b.
A personal history of Drymophloeus in New Guinea.
PALMS 44: 184-186.
Zona, S. 2001a.
Starchy pollen in Commelinoid monocots.
Annals of Botany 87: 109–116.
Zona, S. 2002a.
Roystonea regia.
Enzyklopädie der Holzgewächse III-4, part 26, pp. 1–5.
Zona, S. 2002b.
A revision of Pseudophoenix.
PALMS 46: 19-38.
Zona, S. 2002c.
Name changes in Attalea.
PALMS 46: 132-133.
Zona, S. 2003.
Endosperm condition and the paradox of Ptychococcus paradoxus.
Telopea 10: 179-185.
Zona, S. 2004.
Raphides in palm embryos and their systematic distribution.
Annals of Botany 93: 415-421.
Zona, S. & F. B. Essig. 1999.
How many species of Brassiophoenix?
Palms (formerly Principes) 43: 45-48.
Zona, S, D. Evans & K. Maidman. 2000.
La palma barrigona.
PALMS 44: 85-87.
Zona, S. & K. Maidman. 2001.
Growth rates of palms in Fairchild Tropical Botanic Garden.
PALMS 45: 151-154.
DHTML JavaScript Menu By Milonic.com
Copyright © 2007 Virtual Herbarium - All rights reserved
11935 Old Cutler Road, Coral Gables, FL 33156-4299 USA
Phone: 305-667-1651 • Fax 305-665-8032